John A Schetz
University of North Texas HSC, USA
Title: Unraveling the complex psychopharmacology associated with the adverse neuropsychiatric side effects and recreational use of HIV-1 antiretroviral drugs
Biography
Biography: John A Schetz
Abstract
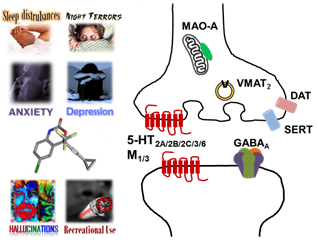
Anti-HIV pharmacotherapies currently remain centralized around HAART. Since its introduction in 1998, efavirenz has been a mainstay of HAART, because it efficaciously suppresses HIV-1, and while it was recently downgraded by DHHS from a first line to an alternative treatment for naïve patients due to neuropsychiatric adverse events (NPAE), both WHO and South African guidelines continue to recommend efavirenz as the preferred NNRTI for HAART in adults and its popularity is likely to continue now that generic forms are becoming available. Only recently, however, has significant progress been made toward a molecular mechanistic appreciation of efavirenz-mediated NPAEs and its attractiveness as a recreational drug. Contributing factors for efavirenz are its ability to rapidly accumulate in brain and a narrow therapeutic window. Our receptor pharmacology studies indicate that within a concentration range relevant to its brain exposure, efavirenz is able to disrupt multiple monoamine neurotransmitter systems, including dopaminergic, serotoninergic, cholinergic, and GABAergic systems, hence its combined effects on these systems is likely to be responsible for some of efavirenz’s NPAEs, such as sleep disturbances, depression, anxiety, hallucinations, dizziness, headaches and memory impairments. Within a concentration range relevant to its brain exposure, a number of CNS off-target interactions for efavirenz were identified, including with the 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, M1, M3 and GABAA receptors, DAT, SERT, and VMAT2 transporters, and MAO-A. In rats trained to discriminate LSD from saline, efavirenz partially substitutes for LSD and this substitution is blocked by pre-treatment with a 5-HT2A receptor selective antagonist. Efavirenz also apparently competes for the same binding site at the 5-HT2A receptor as LSD, and prolonged chronic treatment with efavirenz leads to a pronounced reduction in 5-HT2A receptor levels. Finding from our receptor pharmacology studies with those in animals and humans correlate primarily with behavioral effects related to depressive, anxiogenic, hallucinogenic, and sleep disturbances.